Ultra-high sensitivity detection of bimodal probes at ultra-low noise for combined fluorescence and positron emission tomography imaging
ABSTRACT
Multimodal imaging is quickly becoming a standard in pre-clinical studies, and new developments have already confirmed the strength of acquiring and analyzing parallel information obtained in a single imaging session. One such application is the introduction of an internal reference moiety (e.g. radioisotope) to an activatable fluorescent probe. One of the limitations of this approach consists of working at concentrations which are within the overlapping range of sensitivities of each modality. In the case of PET/Fluorescence imaging, this range is in the order of 10-9 nM. Working in epi-illumination fluorescence imaging at such concentrations remains challenging. Here, we present in vitro and in vivo detection limits of a new fluorescent compound.
1. INTRODUCTION
Multimodal imaging is quickly becoming a standard in pre-clinical studies, and new developments have already confirmed the strength of acquiring and analyzing parallel information obtained in a single imaging session1 . One such application is the introduction of an internal reference moiety (e.g. radioisotope) to an activatable fluorescent probe2 . One of the limitations of this approach consists in working at concentrations which are within the overlapping range of sensitivities of each modality. In the case of PET/Fluorescence imaging, this range is in the order of 10-9 nM. Working in epi-illumination fluorescence imaging at such concentrations remains challenging.
Many incentives are supporting the substitution of PET and SPECT imaging by fluorescence imaging: stability of the probes, cost of the infrastructure, risks of manipulation, and so on. On the other hand, the exquisite sensitivity of PET imaging allows for the detection of low molecular concentration targets, such as receptors. In order for fluorescence imaging to be a proper substitute, optical scanners must be optimized: highly efficient filters, powerful illumination and extremely sensitive camera.
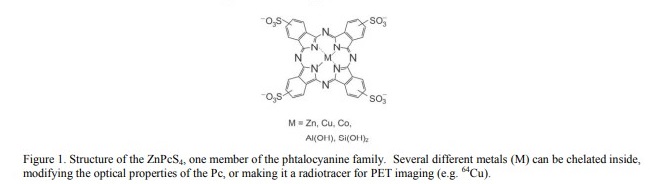
Our center recently published several papers concerning a family of molecules named phtalocyanines (Pc) (Fig. 1), which have potential use in photodynamic therapy (PDT)3 . Phtalocyanines are chelators that can trap a metal (or radiometal), which will affect their physicochemical properties. When a Zn atom is trapped, the molecule becomes highly fluorescent in water and also a very effective light sensitizer for PDT. It is also possible to insert a 64Cu radioisotope instead, which transforms the phtalocyanine into a PET tracer4 . This duality confers a great potential for Pc’s to be used as bimodal probes, i.e. by coinjecting a mixture of both CuPc and ZnPc. While it is possible to use 64CuPc’s to track ZnPc’s, it would be more useful to simply track the ZnPc directly. One of the major issues with this strategy is that some ZnPc’s (e.g. ZnPcS4) have a tendency to form aggregates in vivo, which reduces the fluorescence emission. Hence, to track ZnPcS4, a highly sensitive camera is required.
Here, we characterized the limits of detection of ZnPcS4 and compare it both with a standard commercial fluorophore (Cy7) and against the typical concentration range at which CuPcS4 is used for PET imaging. In order to maximize the sensitivity of our system, we use the EM N2 EMCCD camera by NüVü Cameras. This liquid nitrogen cooled camera, and its recently introduced thermoelectrically cooled version, the HNü, feature very attractive characteristics for ultralow light and ultra-sensitive optical biomedical imaging with: the best SNR in low light applications with the same EMCCD detectors (e2v) as competitors thanks to reinvented CCD readout electronics (the CCD Controller for Counting Photons - CCCP), the lowest clock-induced charges (CIC) providing lowest background noise (<0.001ē/pixel/frame with 512x512 pixels at -85°C), efficient photon counting mode to suppress the excess noise factor (ENF), high charge transfer efficiency (CTE) (up to 0.9999958) to prevent pixel leaking, high EM gain available (up to 5000), and this with no noise-filtering algorithm. Our results indicate that despite the low fluorescence of ZnPcS4 in vivo, we are able to track this molecule at concentrations nearing typical CuPcS4 concentrations, while for comparison, we show that Cy7 can be detected at concentrations one order of magnitude below typical CuPcS4 concentrations.
Для продолжения чтения вы можете скачать полную версию материала по ссылке ниже